What is a Chemistry Chart - Periodic Table

A periodic table, sometimes also referred to as a chemistry chart, is a graphical representation that shows how the chemical elements are arranged according to their atomic number, electron configuration, and chemical properties.
It is a tabular representation of elements where the table is divided into seven rows and 18 columns called periods and groups, respectively. The rows generally have metals on the left side and nonmetals on the right side, whereas the columns contain the elements with identical chemical behavior.
Nowadays, Chemistry Charts are used in various industries such as education, scientific researches and developments, aviation, etc. When used for academic and training purposes, while the students use these charts to get a better understanding of chemicals, the teachers exploit these tabular representations to explain the lessons well. A periodic table is also helpful in establishing the relationship between different chemicals.
The History of Chemistry Chart/Periodic Table
The history of chemistry chart is as old as 1869, when a Russian chemist Dimitri Mendeleev started developing the periodic table by arranging the chemical elements according to their atomic mass, particularly in the lightest to the heaviest order. A few other contributors behind this periodic table concept were Antonie-Laurent de Lavoisier, Johann Wolfgang Dobereiner, John Newlands, Julius Lothar Meyer, Glenn T. Seaborg, etc.
Through the passage of time and the discovery of new elements, the structure of the periodic table has been altered and improved. However, the basic nature of the chart remains unrevolutionized. Dimitri included 63 elements in his first periodic chart that is now known to have 118 elements.
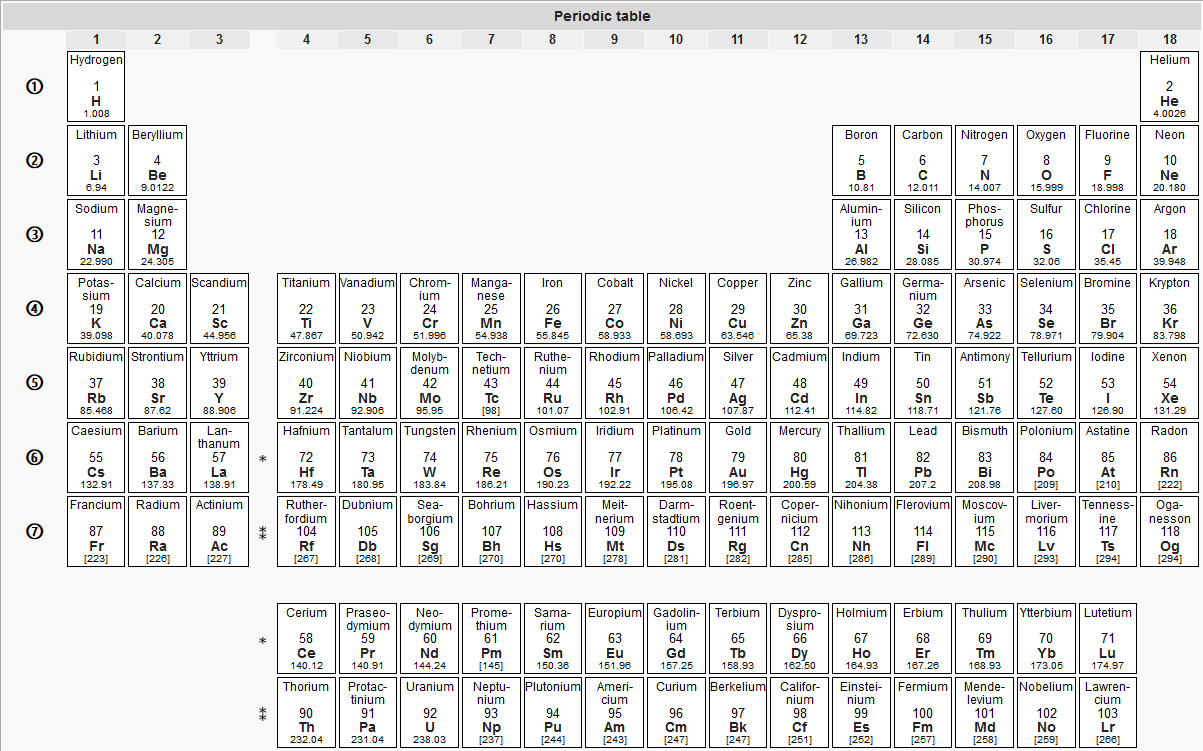
Image source: https://en.wikipedia.org/wiki/History_of_the_periodic_table
Uses of Chemistry Charts in Our Life
Chemistry is an inevitable part of our everyday lives. We begin our day with chemistry, deal with it throughout the day, and wind up everything in the evening.
For instance, chemistry is present in the food we eat, thoughts that come in our minds, things we feel and touch, the water we drink to be alive, and the air we breathe. Our body has many types of chemical compounds that are quite hard for us to believe. Emotions like love, joy, anger, jealousy, etc. are the result of chemical messengers that travel through our veins.
Likewise, onion is a healthy vegetable for us that is completely harmless as long as we don't touch it. But the same onion brings burning sensation and tears in our eyes as soon as we start peeling it.
Another surprising example of chemistry is a soap that is being used by people for quite a long time now. Crude soap is made by humans by mixing animal fats, ashes, oil-based grease, and cleaner. It's quite amazing how something that messy dirty makes us clean. The answer lies in the fact that chemical interactions make every such thing possible.
Even our body comprises several chemical compounds present in many combinations of elements like oxygen, hydrogen, carbon, calcium, phosphorus, and nitrogen. There are chemical messengers known as neurotransmitters that are responsible for all our actions and reactions.
Below are some examples of science charts for your understanding and references:
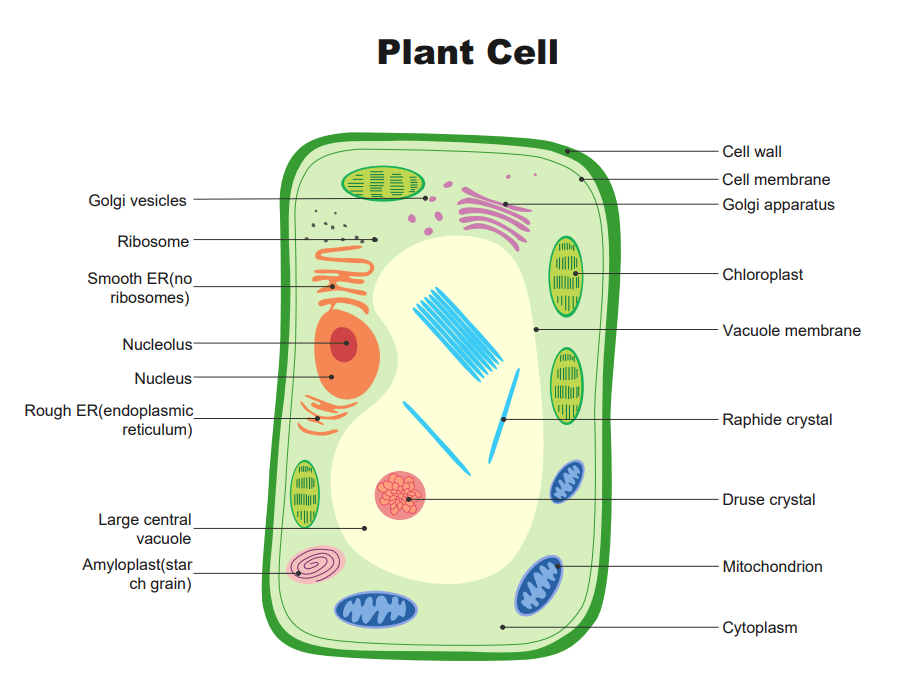
Plant Cell
Plant cells are eukaryotic. This means they have a membrane-bound nucleus and organelles. These microscopic objects are the basic unit of life and belong to a kingdom called Plantar.
The plant cells get support from the cell wall and create sugar through photosynthesis with the help of special organelles called chloroplasts. Animals, fungi, etc. are composed of at least one eukaryotic cell. Similarly, bacteria and archaea are made up of a single prokaryotic cell.
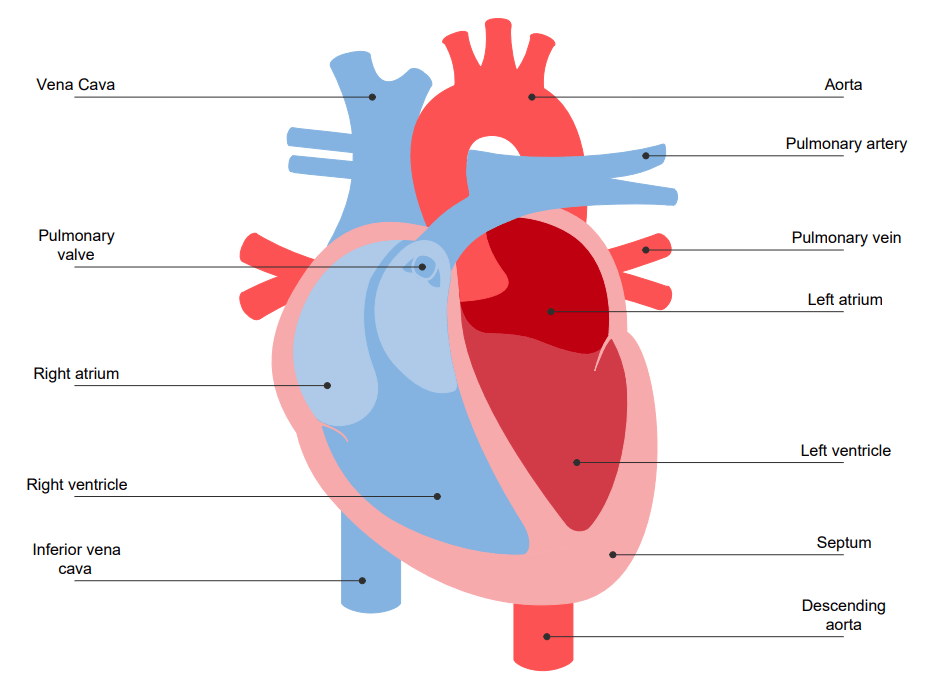
Structure of a Heart
A heart is divided into four chambers, i.e., two atria and two ventricles. While the function of atria is to receive blood, the ventricles are responsible for pumping the blood. The pumped blood is then received by the right atrium from the superior and inferior vena cavas and the coronary sinus. From there, it is further moved to the right ventricle and is then pumped to the lungs.
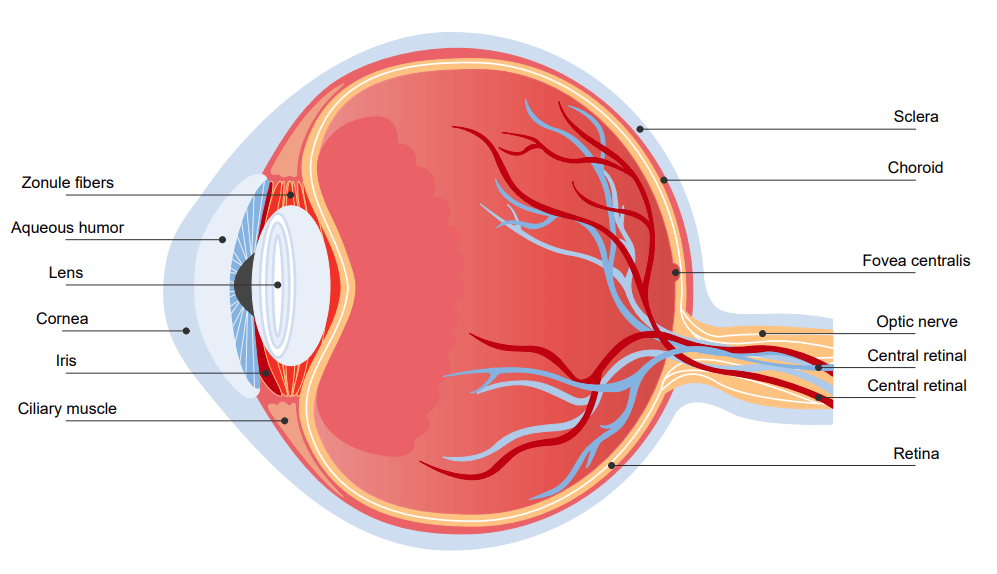
Structure of an Eye
The structure of an eye is quite complex. The eyelids of the eyes automatically contract or expand to adjust according to the amount of light they receive. The orbit is the bony cavity that contains the eyeball, muscles, nerves, blood vessels, and the element that produces tears. The light enters the eyes through the cornea and then travels through the pupil. The iris controls the amount of light entering the eye. Behind the iris is the lens that focuses light onto the retina.
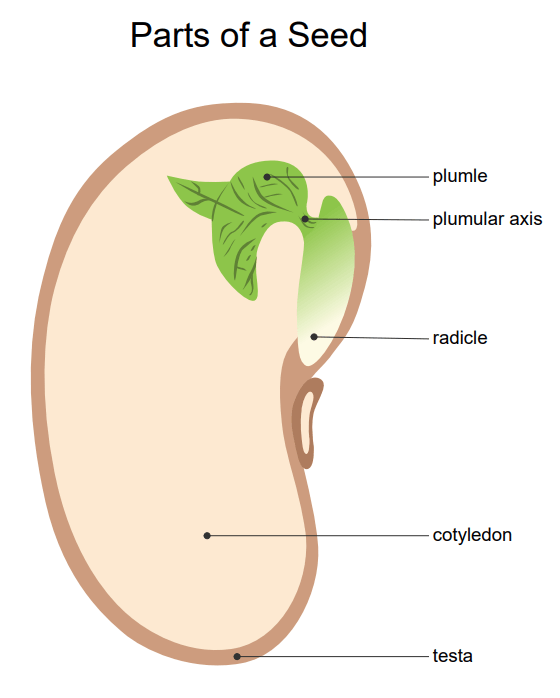
Parts of a Seed
Seeds are primarily divided into three parts, namely the embryo, endosperm, and seed coat. Before emerging, there is a young multicellular organism known as an embryo. The endosperm stores food containing starches, and the seed coat is the protective layer that covers the seed.
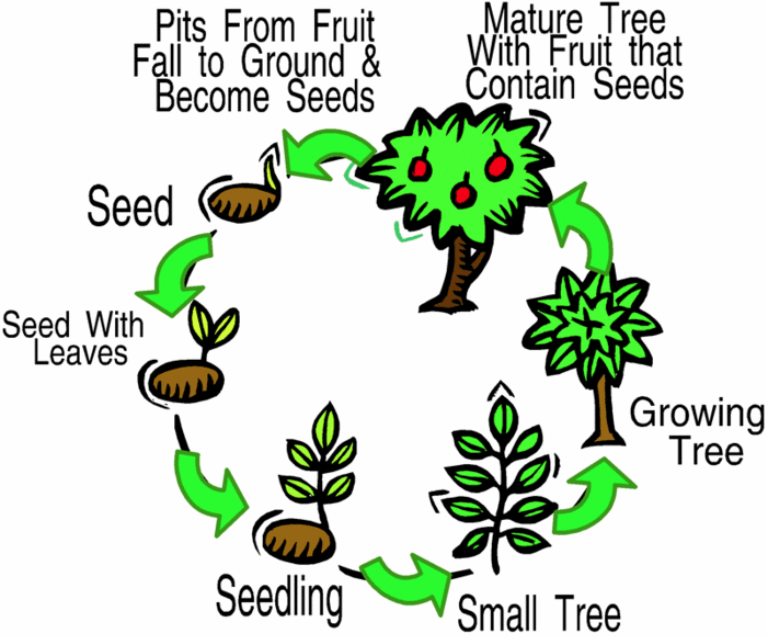
Plant Life Cycle
The life cycle of a plant starts when a seed falls on the ground. The entire plant cycle's major stages are the seed, germination, growth, reproduction, pollination, and seed spreading.
Image source: https://edu.glogster.com/glog/plant-life-cycle/veid3i9d2b
Hence, it would be safe to say that chemistry is an inevitable part of the modern lifestyle and plays a significant role in humans' efficient living. In this entire process, a periodic table has its own importance as it helps in expanding the uses of chemicals depending on their characteristics, thus helping the researches come up with new discoveries now and then.